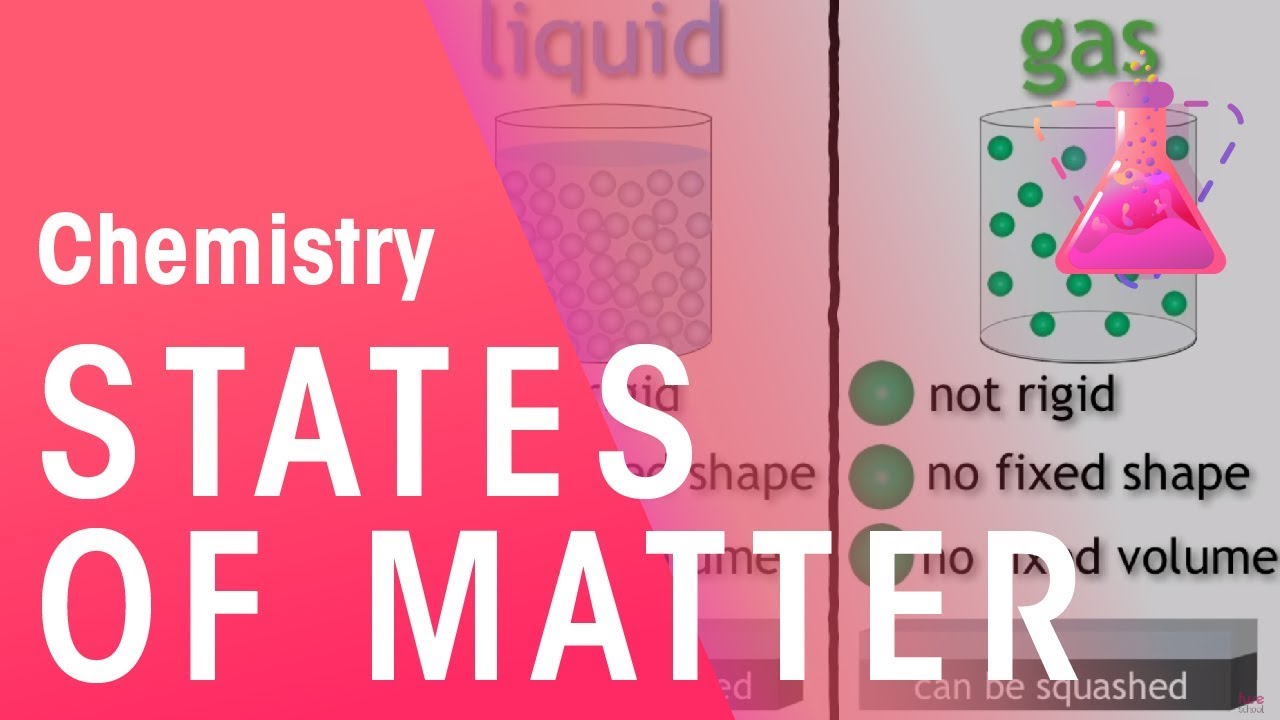
In class we were learning about 3 matters. ( Solid, Liquid, and Gas )
Condensation
Evaporation
Freezing.
What i was learning this was...
Atoms: Atom are the basic units of matter and the defining structure of elements
Molecules: Molecules is the smallest particle in a chemical element or compound that has the chemical properties of that element or compound
Element: An element is a substance consisting of atoms which all have the same number of protons- i.e. the same atom number
Compound: A compound is a substance formed when two or more chemicals elements are chemically bonded together
Mixture: A mixture is a material system made up of two or more different
Chemicals reaction: Chemicals reaction, a process in which one or more substances ( The reactants ) are converted to one or more different substances.
Reactant: A substance participating in a chemical reaction
Product: A product is a substance that is formed as the result of a chemical reaction
Boiling: Boiling is the process by which a liquid turns into a vapour when it is heated to its boiling point
Melting: is a physical process that results in the phase transition of a substanc from a solid to a liquid
Condensation: Condensation is the change of the physical state of matter from gas phase into liquid phase
Evaporation: Evaporation is the process of a substancce oin a liquid state changing to a gaseous state due to an increase in temperature and/ or pressure.
No comments:
Post a Comment